CASE OF THE MONTH: November 2022
Long-standing nyctalopia



History of Present Illness:
- A 65-year-old male with multiple medical co-morbidities is referred for long-standing nyctalopia in both eyes.
Past Medical History:
- HIV (controlled on HAART, complicated by cryptococcal meningitis and cerebral toxoplasmosis)
- Diabetes mellitus type 2
- Hepatitis.
Examination:
- VA 20/30 OD, 20/30 OS
- IOP normal OU
- Anterior segment: normal OU
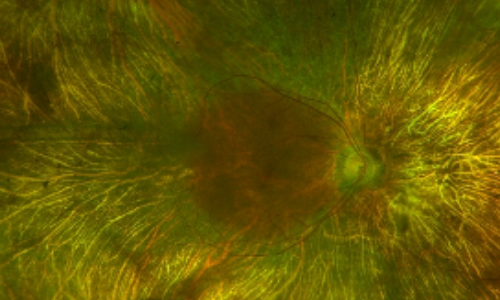
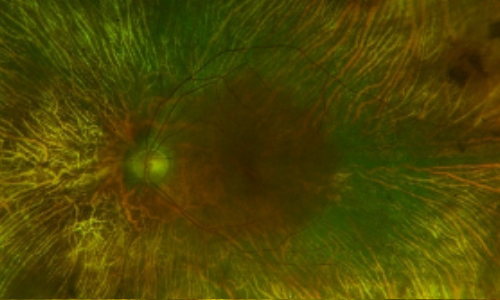
Dilated Fundus Examination /Fundus Photos
Retina evaluation revealed bilateral symmetric pigmentary retinopathy and concentric chorioretinal atrophy anterior to the arcades in both eyes. The macular region was spared in both eyes. There was no vitreous or chorioretinal inflammation present.
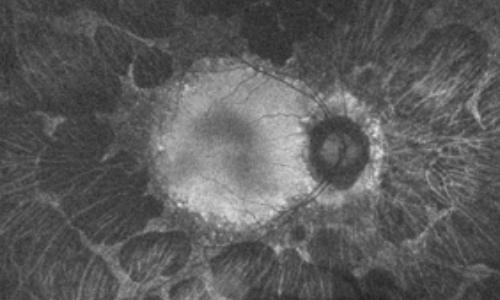
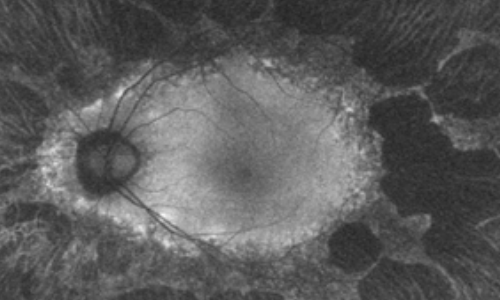
Fundus Autofluorescence Imaging
Fundus autofluorescence revealed drop out peripherally in a scalloped pattern with hyper autofluorescence at the margins of the outer retina atrophy in both eyes.
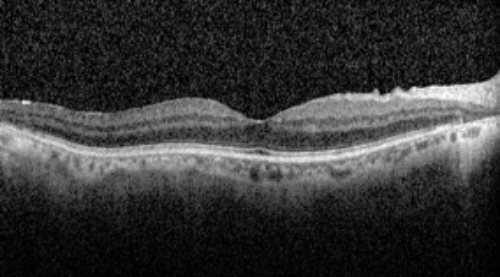
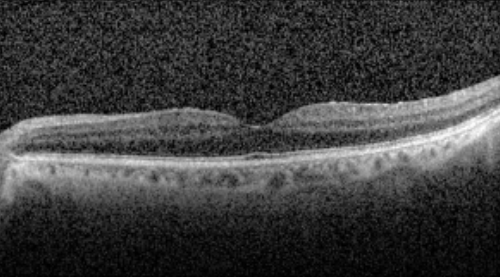
OCT
OCT revealed outer retinal atrophy temporal to the macula with a relatively preserved foveal contour in both eyes.
Further History and Workup
There was no family history of inherited retinal disease.
It was later revealed that the patient previously treated with didanosine (DDI) for an extended period for HIV in the 1990s.
Genetic Testing
Genetic testing revealed a pathological heterozygous variant in the RPGRIP1 gene that is normally expressed in the rod and cone photoreceptors and functions to anchor the RPGR protein within the photoreceptor connecting cilium. Testing was notably negative for mutations in OAT and CHM.
Diagnosis
Didanosine-associated retinal toxicity in a patient with heterozygous RPGRIP1 mutation.
Discussion
This is a case of DDI toxicity, perhaps being unmasked in the setting of genetic predisposition. Importantly, the patient had a long-standing history of DDI use, and retinal features classic for toxicity. Lenis et al described a case of DDI-toxicity in a patient with heterozygous mutation in CRB1, likewise hypothesizing that genetic risk factors may make certain individuals more susceptible to DDI retinal toxicity.
DDI, a purine analogue, is one of the earliest nucleoside reverse transcriptase inhibitors (NRTIs) approved for use in 1991. DDI has been shown to deplete wild-type mitochondrial DNA (mtDNA) and increase mutated mtDNA, thus presenting with retinal toxicity findings similar to other mitochondrial disorders. The resultant reduction in normal functioning mitochondria from DDI and the heterozygous mutation of the RPGRIP1 gene product possibly lowered the disease threshold and interfered with the high metabolic demands, outer segment disk turnover, and the phototransduction cascade.
There is no treatment for the chorioretinal atrophy associated with DDI apart from medication cessation.
Clinical Course
This patient has been observed for over three years without obvious progression on serial imaging.
Summary
This case highlights a potential role for genetic susceptibility to retinal toxicity in DDI-associated retinal toxicity.
References
Gabrielian A, MacCumber MM, Kukuyev A, Mitsuyasu R, Holland GN, Sarraf D. Didanosine-Associated Retinal Toxicity in Adults Infected With Human Immunodeficiency Virus. JAMA Ophthalmol. 2013;131(2):255–259.
Lenis TL, Botsford BW, Sarraf D, Papakostas TD. Didanosine-Associated Retinal Toxicity in a Patient With a Mutation in the CRB1 Gene Journal of VitreoRetinal Diseases. 2022;6(4):329-331.
Roepman R, Bernoud-Hubac N, Schick D, et al. The retinitis pigmentosa GTPase regulator (RPGR) interacts with novel transport-like proteins in the outer segments of rod photoreceptors. Human Molecular Genetics. 2000;9(14):2095-2105.
Wang H, Lemire BD, Cass CE, et al. Zidovudine and dideoxynucleosides deplete wild-type mitochondrial DNA levels and increase deleted mitochondrial DNA levels in cultured Kearns-Sayre syndrome fibroblasts. Biochim Biophys Acta. 1996;1316(1):51-598634344